Early Mortality Syndrome (EMS) as new Emerging Threat in Shrimp Industry
Abstract
Early Mortality Syndrome (EMS) also named Acute Hepatopancreatic Necrosis Disease or AHPND should be considered as a new emerging shrimp disease that has been attacked to shrimp farms in Southeast Asia. It was detected in shrimp farms in southern China as first record in 2009 and then in Hainan Island in 2010 and afterward in Viet Nam and Malaysia in 2011 and consequently in the eastern part of Thailand since 2012 and widely spread to other culture areas of Thailand. Its annual losses are estimated more than USD1 billion. Outbreaks of EMS naturally occur in the first 30 days after stocking a freshly arranged shrimp pond, and rate of mortality can pass beyond 70%. The scientists recently found that (EMS/AHPND) could be initiated by a bacterial agent that termed V. parahaemolyticus is transferred through oral and then localizes the shrimp gastrointestinal tract and create a poison that causes tissue devastation and invalidism of the shrimp digestive system known as the hepatopancreas. So far, no official report was recorded in human cells. Therefore, it is unlikely that the specific strain of V. parahaemolyticus will pose any risk when consumed by human beings. It causes some clinical signs which include pale discoloration and atrophy (size reduction) of hepatopancreas, which appears granular when pressed between the fingers, with occasional black streaks. Other clinical signs include pale and empty stomach and gut, reduced growth, movable shell and black discoloration. Also, lethargy, swimming sluggishly along the dikes, spiral swimming and reduced flourish and feeding are observed in EMS/AHPND. More investigation are needed and should be continue on the development of rapid diagnostic tests for prompt detection of the EMS/ AHPND pathogen that will facilitate developed management of hatcheries and ponds, and could be lead to a long-term elucidation for the disease aspects.
INTRODUCTION
The annual world aquatic animal production has been on growth during last recent decade. These amounts prepare 19Kg of per capita consumption and 17% of animal protein requirements and have a strong roll on food safety. The total aquatic animal produc-tion on 2010 was about 148 million Metric tons that 128 million tons of them had been directly consume by man and their remains used for fish meal and other purposes (FAO, 2013). Although the portion of fish catching is bigger than aquaculture, but aquaculture contribution increased from 13% on 1990 to 40% on 2010. The roll of aquaculture portion in human consumption was about 47% on 2010. This product prepares about 220 million jobs opportunity in dif-ferent branches in the world (FAO, 2013). One of the most important problems in aquatic animal produc-tion especially in aquaculture activity is health and diseases management, so the fish and shrimp farmer lost several millions USD due to diseases each year. According to worldwide extension of aquaculture ac-tivity, new emerged diseases and occurrence of other diseases increased year by year. So far, about twenty viral, four bacterial, three fungal and some protozo-an diseases had been recognized in crustacean and shrimps (Muhammad, et al., 2012). Some important viral diseases consist of WSSD and TSV. Recently, a new emerged lethal disease that termed EMS (Early Mortality Syndrome) or AHPND (Acute Hepatopa-ncreatic Necrosis Disease) had been added to list of shrimp diseases during last recent years.
EPIDEMIOLOGY
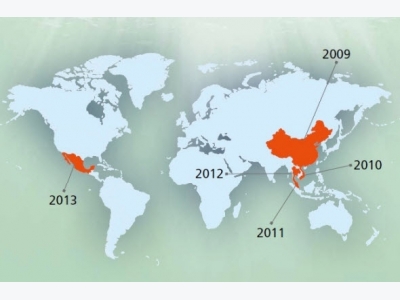
This disease had caused mass mortality in China (2009) as first time and then in Viet Nam (2010), afterward in Malaysia (2011) and finally in Thailand (2012) (Figure 1). It named as EMS due to mass mor-tality during few days after shrimp post larvae stoking. This is an acute disease and affected region in China had lost 80% of their products due to this catastrophic disease. The economic lost was estimated 570000 till 7200000 USD on 2011 and 2012 in Vietnam. This disease decreased the mass production from 70000 tons in 2010 to 40000 and 30000 tons in 2011 and 2012, respectively. About 7% of total shrimp produc-tion lost in Thailand especially in coastal area during 2012 (Lightner et al., 2013). This disease had been re-ported from Western Hemisphere and Mexico during 2013 and made about 118 million USD economic lost in a part of this country (Schryver et al., 2014). The spread to the Western Hemisphere is a major concern to shrimp producers in the region. To date, the only peer-reviewed published method for determining whether mortalities are due to AHPND is through histological examination. A novel PCR detection method was employed to assess samples from Mex-ico in order to confirm the presence of the pathogen in this country. This manuscript details the detection methods used to confirm the presence of AHPND in Mexico. Both immersion and per os challenge stud-ies were used to expose the Penaeus vannamei to the bacteria in order to induce the disease. Histological analysis confirmed AHPND status following the challenge studies. Also provided are the details of the molecular test by PCR that was used for screening candidate V. parahaemolyticus isolates. A rapid PCR assay for detection of AHPND may help with ear-ly detection and help prevent the spread of AHPND to other countries (Nunanr et al., 2014). Despite of trying to disease control during last recent years, it is not under control and made severe mortality in some countries such as Vietnam and Thailand in 2014. Al-though the disease has already been identified and it is transmitted by ingestion is expressed, it seems that the following conditions are included in the incidence and prevalence of disease:
1. High stocking density
2. Sensitivity of species
3. The high salinity of the water in areas near the sea
4. The lack of reservoir pond and water treatment in shrimp farms
5. Lack of attention to process of Pond preparation
6. Stress during transportation or storage of shrimp post larvae
7. Low oxygen conditions8. Feed mismanagement (Ghavampour, 2012)

Figure 1: Geographical distribution of (EMS/AHPND) in the world
ETIOLOGY
After occurrence and spreading of EMS/AHPND in Southeast Asia, initial study had been started and focused on recognition of pathogen. This disease had considered as an unknown pathogen (Idiopathic) article and had been believed several parameter like cypermethrin and other insecticide pollution, envi-ronmental pollution, some parasites, some unknown parameters in feed, harmful algae, probiotics, family cross breeding, etc. and have a critical role in it (FAO, 2013). However, in early 2013, the Aquaculture Pa-thology Laboratory at the University of Arizona was able to isolate the causative agent of AHPND in pure culture. Immersion challenge tests were employed for infectivity studies, which induced 100% mortality with typical AHPND pathology to experimental shrimp exposed to the pathogenic agent. Subsequent histo-logical analyses showed that AHPND lesions were experimentally induced in the laboratory and were identical to those found in AHPND-infected shrimp samples collected from the endemic areas. Bacterial isolation from the experimentally infected shrimp enabled recovery of the same bacterial colony type found in field samples. In 3 separate immersion tests, using the recovered isolate from the AHPND-posi-tive shrimp, the same AHPND pathology was repro-duced in experimental shrimp with consistent results. Hence, AHPND has a bacterial etiology and Koch’s Postulates have been satisfied in laboratory challenge studies with the isolate, which has been identified as a member of the Vibrio harveyi clade, most close-ly related to V. parahemolyticus (Tran et al., 2014).
Regarding to last investigation bacteriophage virus has a potential role in pathogenicity in this disease (Flegel, unpublished data). They could not find any relationship between virus and bacterial pathogenicity by DNA sequencing but they confirmed that the vi-rus presence over the Vibrio parahaemolyticus by TEM (Eduardo et al., 2012). Finally, an especial strain of Vibrio parahaemolyticus that affected by phage virus was responsible of EMS\AHPND (Lightner, un-published data). They identified it as a unique strain of V. parahaemolyticus after affected by bacteriophage virus can produce a kind of toxin that makes this dis-ease (GAA, 2013). An analogous advent occurs in the cholera as human killer disease, where a phage makes the V. cholerae bacterium talented of making a poison that causes cholera’s life-threatening gastroenteritis that may quickly cause dangerous fluid loss in human
Researchers found main pathogen in shrimp farms that transmitted to sensitive host by digestive system and located in the gut and then produced the tox-in that causes necrosis and degeneration especially in haepathopancreas. This disease cannot transmit to human and it is not considered as a man health prob-lem (FAO News, 2013). Although the main pathogen in this disease is V. parahaemolyticus but there is some reports about V. owensii that report it as a causative agent (Shrimp news, 2013).
Also, variation of V. parahaemolyticus isolates from a single Thai shrimp farm that experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND) were investigated and their results revealed the possi-bility of diversity in isolates of V. parahaemolyticus that may cause early mortality in shrimp cultivation ponds in Thailand ( Joshi et al., 2014).
Polecats worm that used for feeding spowner shrimp in hatcheries can transmit the pathogen to hatchery and affect shrimp post larvae (Flegel, unpublished data)
RESISTANCE TO PHYSICAL AND CHEMICAL ACTION
Temperature and Freezing: Attempts to transmit AHPND using infected frozen shrimp have been un-successful. Strains of V. parahaemolyticus in seafood are known to be sensitive to freezing (–18°C to –24°C) and reductions in cultural cells to non-detectable lev-els have been reported after freezing for several weeks. Time of total inactivation depends on the initial num-ber of bacteria and temperature.
Refrigeration: Strains of V. parahaemolyticus in sea-food are known to be sensitive to refrigeration (4°C) and reductions in cultural cells have been reported, but not to non-detectable levels during the storage life of seafood products at this temperature
Heating: Strains of V. parahaemolyticus in seafood are known to be sensitive to heating and reductions in cultural cells to non-detectable levels have been re-ported at 55°C for 5 minutes and 80°C for 1 minute (OIE, 2013) so pelleted feed cannot transmit them due to heath usage in production process (Lightner et al., 2013)
Chemicals/Disinfectants: Susceptible to common disinfectants. No cultural cells after exposure to pH
Survival: Survival to 9 and 18 days in filtered estua-rine water and filtered seawater respectively.Densities of V. parahaemolyticus in seawater are known to be temperature dependent (OIE, 2013).
SuSceptible Specie
This disease had been reported from both two impor-tant cultivated shrimps such as black tiger shrimp (P. monodon) and American white leg shrimp (L. van-namei) in Southeast Asia. This disease also reported from (P. chinensis) in that region. It seems that other species are resistant or less susceptible to EMS\AH-PND (FAO, 2011 and OIE, 2013).

Figure 2: Gross signs of Penaeus vannamei aected by AHPNS (Left) compared to normal shrimp (right)Source: D Lightner &/T. Flegel (1)

Figure 3: Pale atrophied hepatopancreas of juvenile Penaeus monodon from Vietnam with AHPNSSource: D. Lightner (1)
Clinical SignS
This disease specified with mass mortality (up to 100%) 20-30 days after stocking. Most important clinical symptoms consist of lethargy, low speed in growth rate, spiral swimming, as well as empty or interrupted gut. Also infected shrimps constantly re-veal an abnormal hepatopancreas (Emaciated, small, distended, pale or black coloration) (Lightner et al., 2013). Therefore, some of the behavioural changes such as lethargy, swimming sluggishly along the dikes, spiral swimming and reduced preening and feeding are also observed in EMS

Figure 4: Atrophied hepatopancrease indicative of AHPNSSource: D. Lightner (4)
HISTOPATHOLOGY
Hepatopancreas is the main target tissue in this dis-ease. The major lesions should be considered in the hepatopancreas during acute progressive degener-ation of the hepatopancreas with initial decrease of R, B and F-cells followed by an obvious reduction of mitotic activity in E-cells. The development of lesion is noticed from proximal to distal with dysfunction of R, B, F, and finally E-cells, with affected hepato pancreas tubule mucosal cells presenting prominent karyomegaly (enlarged nuclei), and rounding and sloughing into the hepatopancreas tubules (Figure 5). The sloughed hepatopancreas cells prepare a sub-strate for bacterial growth, resulting in massive sec-ondary bacterial infection (putative Vibrio spp.) and complete devastation of hepatopancreas at the fatal phase of the disease. Inter tubular haemocytic ag-gregation and haemocytes encapsulation of necrotic hepatopancreas tubules and melanisation of the more proximal portions of hepatopancreas tubules is seen in some infected shrimp. Also, expanding inefficiency of hepatopacreas due to degeneration and incapacitation of tubular epithelium from beginning to end part of them (OIE, 2013) and (Praveena et al., 2014).

Figure 5: Key diagnostic feature of AHPNS-aected hepatopancreas (HP) with medial sloughing of HP cells (right photo), compared with normal HP (left photo) with intact tubules and distinct F, B and R cells Source: T. Flegel (4)
DISEASE DIAGNOSIS
Although research on rapid diagnosis of the disease continues, clinical signs of the disease can help to in-itial diagnosis to professionals. Specific clinical signs such as soft and dark shell, wasting, anorexia and dis-coloration of the hepatopancreas are the major clini-cal signs (Afsharnasab et al., 2014). Histopathological studies show some changes such as atrophy, discolora-tion and growth of bacteria in the hepatopancreas tis-sue. Rapid diagnosis of this disease and its control has led researchers to combat attempts to develop meth-ods for early diagnosis of this disease, eventually led to the construction of PCR kit for detection of the dis-ease by (Lightner et al., 2013). The main advantages of this new method of diagnosis can be made as follows:• Rapid diagnosis AHPND / EMS• Economical advantage of this method• No need for time-consuming and costly tests of histopathology• Preparation of the test on a large number of shrimp samples (Shrimp news, 2013)According to this method, had made a commercial diagnosis test kite that called IQ2000 (Shrimp news, 2013). This method makes a new, rapid and inexpen-sive method called IQ Plus TM AHPND/EMS Kit (POCKIT) additional to PCR kit to disease diagno-sis. The new method is able to evaluate the hepato-pancreas, stool, post larvae, water and food samples (Simon, 2014).
Also, a new PCR test kit had been made for EMS\AHPND diagnosis (Flegel, 2013). According to this research, acute strain of V. parahaemolyticus genome contains a special gene that it is invisible in other strain. They design their PCR test kit based on these genes. This method contains 2 primer that called AP1 and AP2. In case of EMS\AHPND and acute strain of V. parahaemolyticus these two primers activate and help to case recognition (Shrimp news, 2014).
In June 2014, a new and improved PCR method (called the AP3 primer method) for detection of AH-PND bacteria has been developed from the laboratory test results in screening AHPND bacterial isolates us-ing this AP3 primer method, the team recommended that the previously announced AP1 and AP2 primer methods be replaced with this new method (NACA, 2014)
DISEASE PREVENTION AND TREATMENT
Due to identify the cause of disease and its bacteri-al agent possibility of authorized types of antibiotics are effective against Gram-negative bacteria are avail-able but some limitation such as antibiotic resistant, public health problems disease prevention and health management are the best methods for disease control. On the other hand antibiotic usage killed the useful bacteria in the pond that increased disease potency (Schryver, et al., 2014)
Notes in Shrimp Hatcheries
• Pay attention to health, water and feed manage-ment for high quality and health shrimp post lar-vae production as the first way to disease control (Afsharnasab et al., 2014).
• Continual control of different parts of hatchery and shrimp larvae for detection of vibrio bacte-ria especially V. parahaemolyticus for decreasing of bacterial load and disease prevention (MAC, 2013).
• Post larvae health control and certified by respon-sible organs for presence of vibrio and other or-ganisms and their quality control (MAC, 2013 and Shrimp news, 2013).
• Attention to faster growth rate ability and in-breeding, caused disease sensitive production (Shrimp news, 2013).
• Also eye stalk oblation in hatcheries increase the product quantity but decrease the quality and dis-ease tolerance (Shrimp news, 2013).
Notes in Shrimp Farms
• Full compliance with the principles of pond prepa-ration (washing, spraying lime, plowing, etc.) (Af-sharnasab et al., 2014 and MAC, 2013).
• Due to the increasing incidence of disease in high pH water, decreasing or pH control can be consid-ered as a strategy for disease prevention. However, in this case, hydrogen sulfide toxicity possibility increases (Afsharnasab et al., 2014 and Shrimp news, 2013).
• The use of probiotic containing Bacillus in the soil during the pond preparation and during the cul-ture period for the acidic environment can useful for disease control (MAC, 2013).
• Stocking of healthy and high quality post larvae and preparing them from good and authentic hatcheries (Afsharnasab et al., 2014 and Shrimp news, 2013).
• Avoiding from high stocking density for stress de-creasing and diseases prevention (Afsharnasab et al., 2014).
• Continuous soil and water sampling and testing for detection of infectious Vibrio (MAC, 2013).
• Use from high quality and safe food and immune stimulant material for improving shrimp immuni-ty (Afsharnasab et al., 2014).
• Reduction of environmental stress with optimum water and feeding management (MAC, 2013 and Shrimp news, 2013).
• Use of water storage ponds (reservoir) (Muham-mad, et.al, 2012).
• Reducing and keeping water salinity (based on observations of low incidence in low salinity) (Af-sharnasab et al., 2014).
• Use from aeration in ponds and prevention from oxygen drop and stress (MAC, 2013).
• Reporting to responsible institutions in case of mortality during first 45 days after stocking for dead shrimp sampling and testing (MAC, 2013).
• In case of disease water must disinfect with high dose chlorine and dead shrimp must keep in 60oC for minimum 15 minutes after collection (MAC, 2013).
• Utilizing the Biofloc techniques significantly in-creased the survival rate of infected farms (FAO, 2013 and Seafood Source News, 2013)
• Using the poly culture system(shrimp and filter feeding marine fish) prevent from disease and\or increase the survival rate in affected system (Tran et al., 2014) for some reasons as follow:
1. Zooplanktons use from the bacteria as feed and decrease the bacterial load in water and fish by zooplanktons feeding decrease the bacterial total count indirectly
2. Blue-green algae population decrease due to fish feed and after that decrease the bacterial popula-tion
3. The antibacterial effect of fish mucosa decrease the density of bacteria in the ponds (FAO, 2013).
LAST STATUS OF EMS\AHPND IN IR. IRAN
V. parahaemolyticus is one of the natural bacterial floras in Iran’s costal area. Fortunately, there isn’t any official report about occurrence or distribution of mentioned disease in Iran. There is some similarity between Iran and affected area such as: same species, shrimp culture complex are near the sea, high water salinity, Inacces-sibility to fresh water, high pH and temperature, in-complete pond preparation and increases the disease incidence. For these reasons government association must increase their activity especially on live animals import and transporting (quarantine policy, health certificate and biosecurity operations) (Afsharnasab et al., 2014). In comparison Indonesia could be con-sidered as free of this disease due to live animal im-port limitation (Lightner, unpublished data).
CONCLUSION
(Early mortality syndrome) or (Acute Hepatopan-creatic Necrosis Disease), (EMS/AHPND) is a new emerging shrimp disease that affected several coun-tries in eastern and western hemisphere such as Chi-na, Vietnam, Malaysia, Thailand and Mexico from 2009. Economic losses of this disease were estimated more than 1bilion US$ in this countries and they lost about 80% of their products in some regions. As re-gards, this is a new emerging disease so initial inves-tigation had been started and focused on recognition of causative pathogen. Finally, Dr. Lightner identified it as a unique strain of V. parahaemolyticus after af-fected by bacteriophage virus that can be produced a kind of toxin that makes this disease. They found the causative pathogen that can be transmitted to sensitive hosts by digestive system and located in the gut and then produced the toxin that causes necrosis and degeneration especially in haepathopancreas and death was happen due to this function . This disease had been reported from P.monodon, L.vannamei and P. chinensis in Southeast Asia and Mexico.
According to current study Hepatopancreas should be considered as the target tissue in this disease. This dis-ease specified with mass mortality (up to 100%) 20-30 days after stocking. Clinical signs consist of slow growth, corkscrew swimming, loose shells, pale col-oration, empty or interrupted gut. Affected shrimps also consistently show an abnormal hepatopancreas (shrunken, small, swollen, discoloration or black col-oration) (Lightner et al., 2013). Affected shrimp sank to the pond bottom and die there. Affected hepato-pancreas doesn’t squash easily by finger (Lightner, 2012). Presence of some microbes including Vibrio spp, microsporidians and nematode has been observed in some samples (Eduardo et al., 2012). The affected shrimps are smaller than healthy one in size.
Histological examination showed the following le-sions: Lack of mitotic activity in generative E cells of the HP, dysfunction of central hepatopancreatic B, F and R cells, karyomegaly and massive sloughing of central HP tubule epithelial cells, massive inter tu-bular hemocytic aggregation followed by secondary bacterial infections (Eduardo et al., 2012), Expanding inefficiency of hepatopacreas due to degeneration and incapacitation. Although clinical signs of the disease can help to initial diagnosis to professionals but histo-pathological studies are the best and routine diagnosis tests. For rapid diagnosis of this disease several PCR kites were designed and produced till now. These PCR kites contain AP1, AP2 and/or AP3 primer for detec-tion of specific acute gene in the bacterial genome. These techniques are faster, more special and cheaper than others.
(EMS/AHPND) should be assumed as bacterial disease and it caused by V. parahaemolyticus, and sev-eral parameters have positive roll on its happen and spreading such as: high stocking density, species sen-sibility, high water salinity, lack of reservoir pond and water treatment in grow out pond, lack of attention to the process of ponds preparation, post larvae poor quality, stress problem specially during transportation and stocking, low oxygen condition, high water pH and weak feeding management.
According to the pathogen property, using from an-tibiotics is suitable way but some public health prob-lems (drug residual) make it useless. So, prevention from pathogen and reducing the risk factors could be nominated as the best actions (goal).
Restricting in the transportation of alive shrimp and good quarantine management will reduce the disease transports risk. High qualified pond preparation (dry-ing, washing, spraying lime, plowing, etc.) are basic activities to reduce the disease transfer risk such as EMS. Using of reservoir and treatment pond can re-duce the disease incidence by chemical materials and\or time spending. Since the high pH can increase the EMS prevalence, the water pH control and reduce the shrimps gut pH can decrease the disease occurrence. Bacillus content probiotic usage increases the water pH and control the disease as above, meanwhile bacillus concentrate can control the water Vibrio popu-lation.
Aeration system can control the DO concentration in the pond and reduce the shrimp stress and disease incidences. Also, good feed management reduces the bottom sediment and increases their conditions to control of stress and diseases. High quality feed spe-cially immunostimulant enriched feed, can help to disease prevention. Utilizing the Biofloc techniques significantly increased the survival rate of infected farms (FAO, 2013 and Seafood Source News, 2013). Alongside above materials for controlling the disease using the poly culture system (shrimp and filter feed-ing marine fish) could be useful in diseases prevention and\or increase the survival rate in affected system. It is necessary to stocking the pond with high quali-ty and high health shrimp post larvae with authentic health certificate.
A good relationship between private sectors (farms and hatcheries), governmental council and profes-sional laboratory as vital triangle can help to disease control. In case of occurrence of suspicious mortality it must be reported to any responsible organs as soon as possible and must increase biosecurity actions in the affected farms and quarantine affaires would be done immediately.
In case of disease observation in region first of all farms water must be disinfected with high dose chlo-rine and then dead shrimp must kept in 60oC for minimum 15 minutes after collection (MAC, 2013).
Fortunately, there isn’t any official report about occur-rence or distribution of mentioned disease in I.R. Iran but concerning to identification of V. parahaemolyticus as a natural flora and presence of L.vannamei as main cultivated species in the country, so it is possible to oc-cur of EMS in the future. It seems restriction in alive shrimp importation and control actions have critical roles in disease control. Also pH control in grow out pond (the pH of pond normally are high in Iran), bi-osecurity activities in farms and hatcheries, stocking with high quality and health post larvae, reduce the stress, more attention to native species, use from high quality feed and immunostimulant enriched feed, good water and feed management, using of reservoir ponds and reinforcement of health supervision and developing of health laboratory can help to disease prevention in I.R. Iran.
Related news
 Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae)
Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae) Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon (Decapoda: Penaeidae): Fine struct
 Microsporidian Impacts Shrimp Production – Industry Efforts Address Control Not Eradication
Microsporidian Impacts Shrimp Production – Industry Efforts Address Control Not Eradication Enterocytozoon hepatopenaei (EHP), a microsporidian parasite that has been widely found in Asia and other parts of the world
 Determination of the infectious nature of the agent of acute hepatopancreatic necrosis
Determination of the infectious nature of the agent of acute hepatopancreatic necrosis Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp