Microsporidian Impacts Shrimp Production – Industry Efforts Address Control Not Eradication
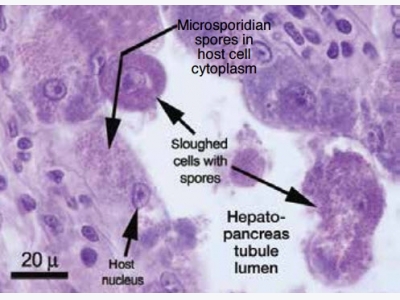
EHP spores infect the tubules of the hepatopancreas in shrimp, which damages the ability of the animals to gain nutrition from feed. Photo by Tim Flegel.
Summary:
Enterocytozoon hepatopenaei (EHP), a microsporidian parasite widely found in Asia and other areas, is impacting aquaculture by severely retarding the growth of cultured shrimp. EHP infects the tubules of the hepatopancreas in shrimp, which damages the organ’s ability to gain nutrition from feed. Fight-ing EHP entails improved biosecu-rity in the hatchery and proper pond preparation and growout management. Total elimination of microsporidians may not be possi-ble. The best approach is to lessen the loads coming into ponds and control their levels.
Enterocytozoon hepatopenaei (EHP), a microsporidian parasite that has been widely found in Asia and other parts of the world, is impacting aquaculture pro-duction by severely retarding the growth of cultured shrimp.
Although the most common pathol-ogy associated with microsporidians is a whitish discoloration in muscles due to spores that can stunt growth and cause other types of problems, EHP is differ-ent. It only infects the tubules of the hepatopancreas in shrimp, which dam-ages the ability of this critical organ to gain nutrition from feed. It is widely understood that EHP does not cause mortality but heavily limits growth. Formerly classified as protozoa, genomic taxonomy has determined that microsporidians are closely related to fungi. About 100 genera of microsporidians are known to infect crustaceans and fish.
EHP is now endemic throughout China, Malaysia, Thailand, Indonesia and Vietnam, and likely present in India and possibly Mexico. It can likely be found anywhere that has imported live feeds from China and animals from areas where EHP is endemic. EHP is very dif-ficult to eradicate. More than likely, we will only be able to control its levels.
How Is EHP Detected?
The pathogen can be detected using gene-based tools such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification testing of feces from broodstock. These methods can also be used with postlarvae. Light micros-copy can be used, as well, although it can be very difficult to visualize the very small spores. Although effective, screening broodstock entails examination of indi-vidual animals, a costly practice. In some areas, there may be no animals totally free of the pathogen.
EHP Treatment
Microsporidian infections are typi-cally treated with a specific class of drugs that is unlikely to be effective against EHP because of its target tissue specific-ity. Dealing with the problem entails a three-prong strategy incorporating biose-curity in the hatchery, proper pond prep-aration and proper pond management during the growth cycle.
Total elimination of microsporidians may not be possible. The best approach is to lessen the loads coming into ponds and control the levels the ecosystem allows. The direct vector of EHP in ponds has not yet been identified.
Biosecurity In Hatcheries
Proper practices and procedures for biosecurity in hatcheries can help control EHP.
No Live Feeds
Pond-reared adult broodstock, as well as those fed infected live feeds, can be infected and spread EHP through feces. The use of live animals -- including poly-chaetes, clams, warmwater squid and locally produced Artemia – in broodstock maturation facilities poses a significant biosecurity risk and should be discour-aged. Live feeds such as krill do not pose a risk. If live feeds are used, they should be frozen, pasteurized or even irradiated.
Disinfection
Maturation facilities and hatcheries should be dried out completely, washed and then disinfected with a caustic solu-tion of sodium hydroxide. It has been suggested that all equipment, pipes and tanks should be soaked in a 2.5% sodium hydroxide solution for at least three hours. After this, the remaining caustic solution should be washed away, and all of the treated materials allowed to dry for an extended period.
Rinse before use with acidified chlo-rine at 200 ppm and pH less than 4.5. Microsporidian spores are extremely resis-tant to most treatments, and complete elimination will prove challenging. The goal is to substantially lower the load.

This image, captured by a transmission electron microscope, identifies the presence of EHP spores. Since this type of microscope uses electrons in the place of light and elec-tromagnets in the place of glass lenses, it allows much higher resolution than traditional microscopes achieve. Photo by Tim Flegel.
Clean Eggs, Nauplii
Proven strategies for washing and rinsing nauplii with the appropriate mix of freshwater and chemicals (iodine and formaldehyde, among others) that can weaken the passive attachment of spores to eggs and nauplii – thus lessening transmission – must become routine. This is an effective tool against EHP, as well as for lowering the loads of the bac-teria that cause early mortality syndrome that pass from broodstock to postlarvae.
Pond Preparation
High organic loads typically relate to spore loads. There is likely some interme-diate vector, and until we are sure what it is, use strategies to properly treat sedi-ments before stocking.
As spores typically are resistant to a wide variety of environmental conditions, with different species displaying differen-tial susceptibility, the general suggestions are to physically remove accumulated organic matter and treat pond bottoms with a very caustic material to bring the pH to 12 and kill many of the spores. Killing all of them may not be possible.
It has been recommended that earthen ponds be disinfected with very heavy use of calcium oxide, or quick lime, applied at a level of 6,000 kg/ha or greater. Pond bottoms must be com-pletely dry. Plow the quick lime into the dried sediments to a depth of 10 to 12 cm, then moisten the sediments to acti-vate the lime. If the application is done properly, the pH of the soils will rise to 12 or more within days and then gradu-ally return to normal as the quick lime becomes calcium carbonate.
Pond Management
After the soils have recovered, use suitable commercial products from the early stages of culture to prevent the accumulation of large amounts of organic matter. These can be used alone or in combination with water exchange.
The goal is to lessen the amount of accumulated organic matter and thus reduce the potential reservoir for spores that will be ingested and continue to infect shrimp. Consistent use at levels that lessen the amount of organic matter is important
Perspectives
Reducing the load of spores in the production environment by reducing their false vertical transmission as a result of contaminated surfaces due to spawn-ing, in combination with aggressively limiting the reservoirs for spores, will reduce the severity of EHP. Consistent use of these methods should lessen the long-term impacts and reduce the envi-ronmental load of spores.
Editor’s Note: Dr. Tim Flegel kindly pro-vided the following additional comments regarding detecting EHP, as well as the photos that illustrate this article.
Microscopy can be used to detect EHP with hepatopancreas tissue smears or tissue sections, but scanning using at least a 40x objective is needed, followed by confirmation by inspection using a 100x objective. Since this is very tedious and also depends on see-ing mature or nearly mature spores, the pro-cess can take a lot of time and may fail if no spores are seen. This often happens with
Litopenaeus vannamei.
Thus, PCR detection is preferred, using DNA extracted from hepatopancreas tissue as the best option. With nested PCR, DNA from feces can also be used, but may give negative results if the tested shrimp have light infections.
Related news
 Analyzing the hydrostability of shrimp feeds
Analyzing the hydrostability of shrimp feeds Aquafeeds can lose nutrients rapidly after immersion, so it is important that feeds have adequate stability and attractability after immersion in water
 The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome
The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome in whiteleg shrimp Penaeus (Litopenaeus) vannamei
 Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae)
Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae) Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon (Decapoda: Penaeidae): Fine struct