Separate Hydrogen and Oxygen from water through electrolysis
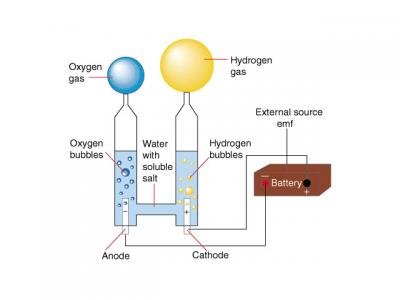
Electrolysis a method of separating elements by pushing an electric current through a compound. It is used in various industrial applications such as removing copper from its ore. It is also used to separate hydrogen and oxygen from water. Electrolysis isn't the most efficient way to obtain hydrogen, but it is one of the easiest and cheapest ways to "homebrew" hydrogen.
Hydrogen is the most abundant element in the universe. With the "green-energy" craze and talk of powering our future oil-free economy on hydrogen, it has gotten much attention in the last few years. Learning about this potential fuel of the future is important and interesting. Besides, hydrogen is a powerful fuel, and blowing stuff up in the name of science is fun.
This section is an explanation of the electrolysis of water, feel free to skip it if you don't find it interesting.
2H2O(l) = 2H2(g) + O2(g)
As everyone knows a water molecule is formed by two elements: two positive Hydrogen ions and one negative Oxygen ion. The water molecule is held together by the electromagnetic attraction between these ions. When electricity is introduced to water through two electrodes, a cathode (negative) and an anode (positive), these ions are attracted to the opposite charged electrode. Therefore the positively charged hydrogen ions will collect on the cathode and the negatively charged oxygen will collect on the anode.
When these ions come into contact with their respective electrodes they either gain or lose electrons depending on there ionic charge. (In this case the hydrogen gains electrons and the oxygen loses them) In doing so these ions balance their charges, and become real, electrically balanced, bona fide atoms (or in the case of the hydrogen, a molecule).
The reason this system isn't very efficient is because some of the electrical energy is converted into heat during the process. There have been reports of 50%-70% efficiency, but I doubt that is possible in a home environment. Anyway, enough with the boring stuff, lets go make some gas!
Related news
 Dissolved Oxygen
Dissolved Oxygen What is dissolved oxygen? How is dissolved oxygen measured? What is the significance of dissolved oxygen? Guidelines for Interpretation of Dissolved Oxygen
 HN diagnoses fish death causes
HN diagnoses fish death causes The recent mass fish deaths in several lakes in Hà Nội were in part caused by untreated wastewater discharge and weather changes, the city’s People’s Committee
 Garlic injection could tackle tree diseases
Garlic injection could tackle tree diseases Injecting trees with a concentrated form of garlic might help save trees in the UK from deadly diseases. Widespread use of the injection process is impractical